Module 1: Chapter 1: Matter in Our Surroundings
Introduction to Matter
introduction-to-matter
Physical Nature of Matter
physical-nature-of-matter
Particles Have Space Between Them
particles-have-space
Particles Attract Each Other
particles-attract
States of Matter
states-of-matter
Change of State: Effect of Temperature
change-of-state-temperature
Sublimation and Effect of Pressure
sublimation-pressure
Evaporation
evaporation
Summary & Practice Questions
summary-questions
Module 2: Chapter 2: Is Matter Around Us Pure?
Pure Substances & Mixtures
pure-substances-mixtures
Types of Mixtures
types-of-mixtures
What is a Solution?
what-is-solution
Concentration of Solutions
concentration-of-solutions
Suspensions & Colloids
suspensions-colloids
Separation Techniques - Part 1
separation-techniques-1
Separation Techniques - Part 2
separation-techniques-2
Crystallisation
crystallisation
Physical & Chemical Changes
physical-chemical-changes
Elements & Compounds
elements-compounds
Chapter 1: Matter in Our Surroundings
Particles Have Space Between Them
Characteristics of Particles of Matter
1️⃣ Particles Have Space Between Them
From Activities 1.1 and 1.2, we observed that particles of:
- Sugar
- Salt
- Dettol
- Potassium permanganate
...got evenly distributed in water.
Everyday Examples
When we make:
- ☕ Tea
- ☕ Coffee
- 🍋 Lemonade (Nimbu Paani)
Particles of one substance get into the spaces between particles of another.
This proves there is enough space between particles of matter!
2️⃣ Particles Are Continuously Moving
🧪 Activity 1.3: Incense Stick Experiment
| Condition | Observation |
|---|---|
| Unlit stick | Need to go very close to smell it |
| Lit stick | Can smell it from a distance! |
Why? The heated particles move faster and spread throughout the room.
🧪 Activity 1.4: Ink in Water (Diffusion)
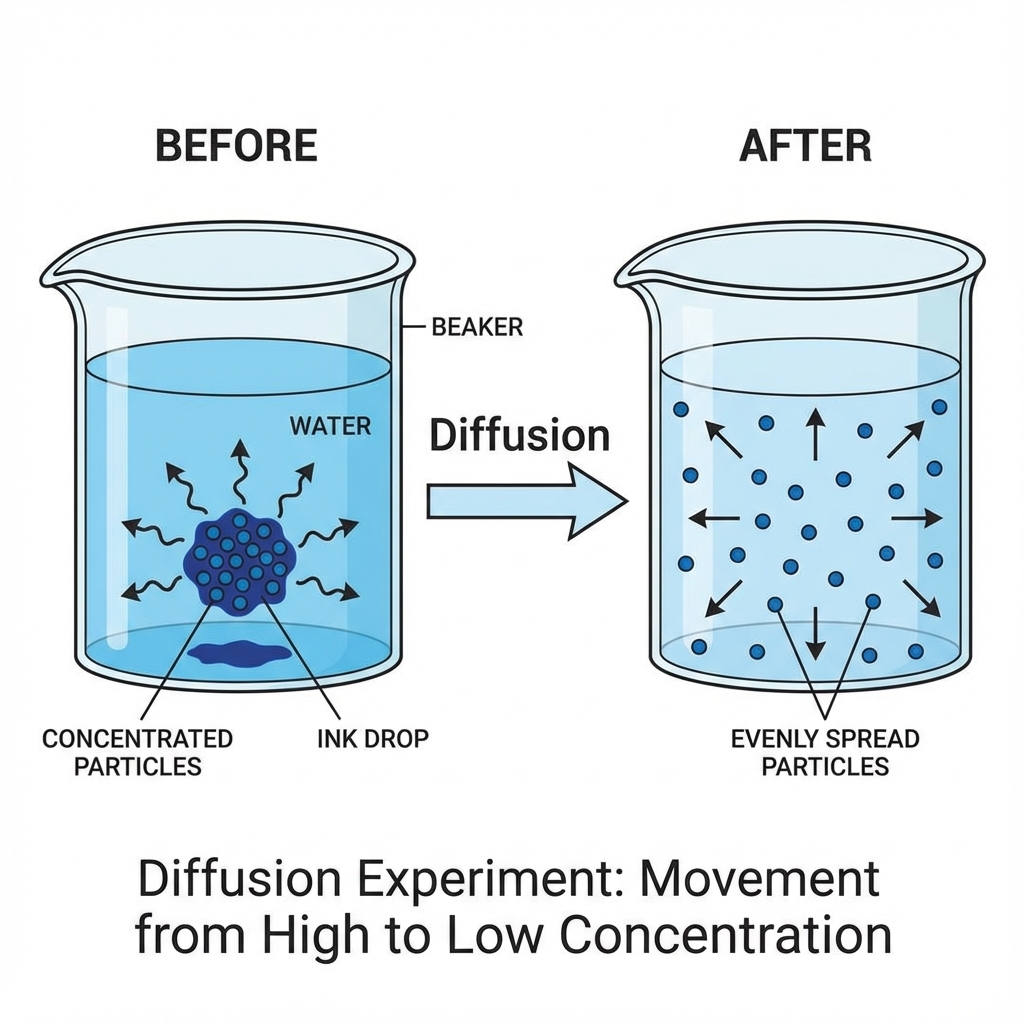
Procedure: Drop ink slowly into a beaker of water (don't stir)
Observation:
- Immediately: Ink stays concentrated
- After hours/days: Colour spreads evenly
Key Concept: Diffusion 🌀
Diffusion is the intermixing of particles of two different types of matter on their own.
Important: Diffusion becomes faster with higher temperature because particles move faster!