Module 1: Chapter 1: Matter in Our Surroundings
Introduction to Matter
introduction-to-matter
Physical Nature of Matter
physical-nature-of-matter
Particles Have Space Between Them
particles-have-space
Particles Attract Each Other
particles-attract
States of Matter
states-of-matter
Change of State: Effect of Temperature
change-of-state-temperature
Sublimation and Effect of Pressure
sublimation-pressure
Evaporation
evaporation
Summary & Practice Questions
summary-questions
Module 2: Chapter 2: Is Matter Around Us Pure?
Pure Substances & Mixtures
pure-substances-mixtures
Types of Mixtures
types-of-mixtures
What is a Solution?
what-is-solution
Concentration of Solutions
concentration-of-solutions
Suspensions & Colloids
suspensions-colloids
Separation Techniques - Part 1
separation-techniques-1
Separation Techniques - Part 2
separation-techniques-2
Crystallisation
crystallisation
Physical & Chemical Changes
physical-chemical-changes
Elements & Compounds
elements-compounds
Chapter 1: Matter in Our Surroundings
Particles Attract Each Other
3️⃣ Particles of Matter Attract Each Other
Force of Attraction Between Particles
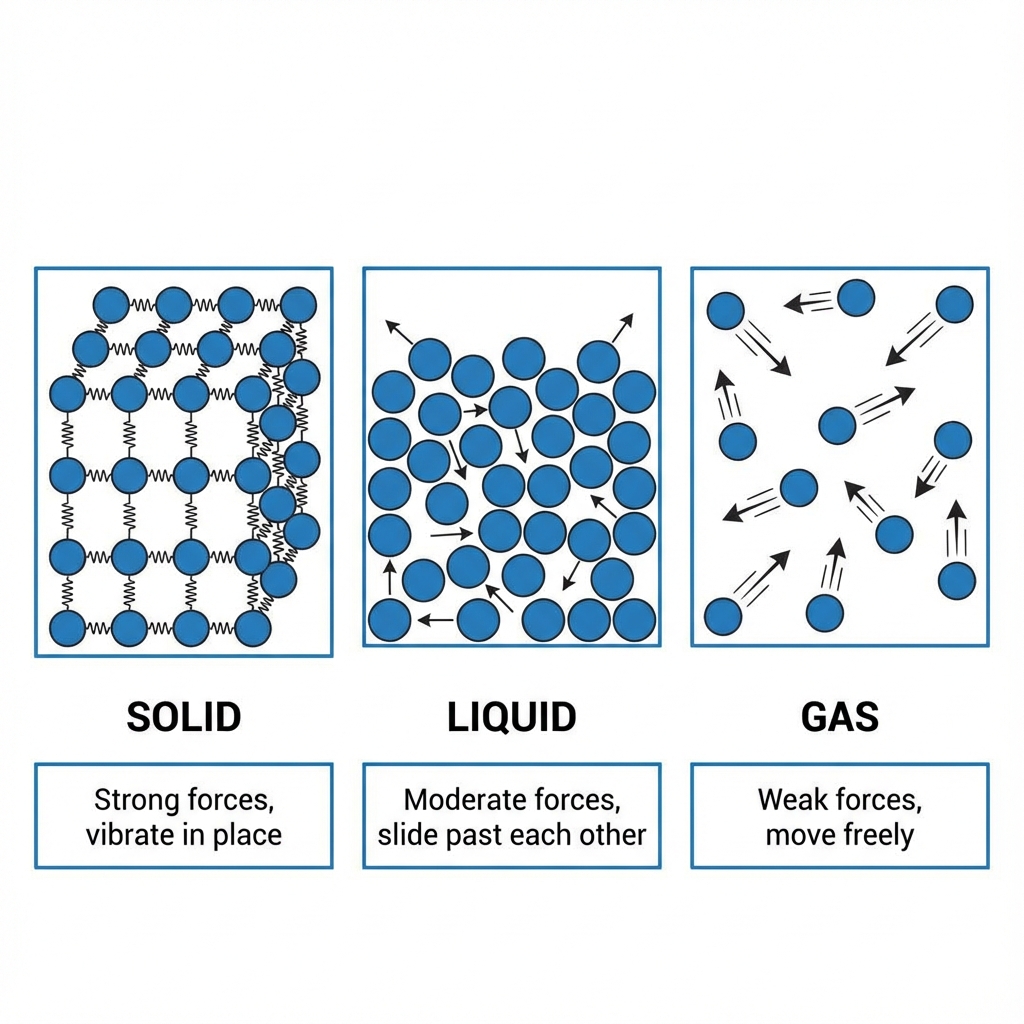
Different substances have different strengths of attraction between their particles.
🧪 Activity 1.6: Human Chain Game
Form three human chains:
- Chain 1: Lock arms like dancers (strongest bond)
- Chain 2: Hold hands
- Chain 3: Touch only with fingertips (weakest bond)
Another group tries to break each chain.
Result: Chain 3 (fingertips) breaks easiest!
Conclusion: The strength of attraction between particles varies in different substances.
🧪 Activity 1.7: Breaking Materials
Try breaking:
| Material | Ease of Breaking | Force Between Particles |
|---|---|---|
| Iron nail | Very difficult | Very strong |
| Chalk | Moderate | Medium |
| Rubber band | Easy to stretch | Weaker |
🧪 Activity 1.8: Cutting Water
Try to cut the surface of water with your fingers.
Observation: You cannot "cut" water — the surface stays together!
Reason: There are forces of attraction between water particles that keep them together.
Summary: Three Characteristics
| Characteristic | Description |
|---|---|
| Space | Particles have space between them |
| Movement | Particles are continuously moving (kinetic energy) |
| Attraction | Particles attract each other with varying strength |
❓ Check Your Understanding
-
Which of these are matter?
- Chair ✅
- Air ✅
- Love ❌
- Smell ✅
- Thought ❌
-
Why does hot food smell reach you faster than cold food?
Hot particles have more kinetic energy and diffuse faster!