Module 1: Chapter 1: Matter in Our Surroundings
Introduction to Matter
introduction-to-matter
Physical Nature of Matter
physical-nature-of-matter
Particles Have Space Between Them
particles-have-space
Particles Attract Each Other
particles-attract
States of Matter
states-of-matter
Change of State: Effect of Temperature
change-of-state-temperature
Sublimation and Effect of Pressure
sublimation-pressure
Evaporation
evaporation
Summary & Practice Questions
summary-questions
Module 2: Chapter 2: Is Matter Around Us Pure?
Pure Substances & Mixtures
pure-substances-mixtures
Types of Mixtures
types-of-mixtures
What is a Solution?
what-is-solution
Concentration of Solutions
concentration-of-solutions
Suspensions & Colloids
suspensions-colloids
Separation Techniques - Part 1
separation-techniques-1
Separation Techniques - Part 2
separation-techniques-2
Crystallisation
crystallisation
Physical & Chemical Changes
physical-chemical-changes
Elements & Compounds
elements-compounds
Chapter 1: Matter in Our Surroundings
Change of State: Effect of Temperature
Can Matter Change Its State? 🔄
Yes! Matter can exist in different states. For example, water can be:
- 🧊 Solid (ice)
- 💧 Liquid (water)
- 💨 Gas (water vapour/steam)
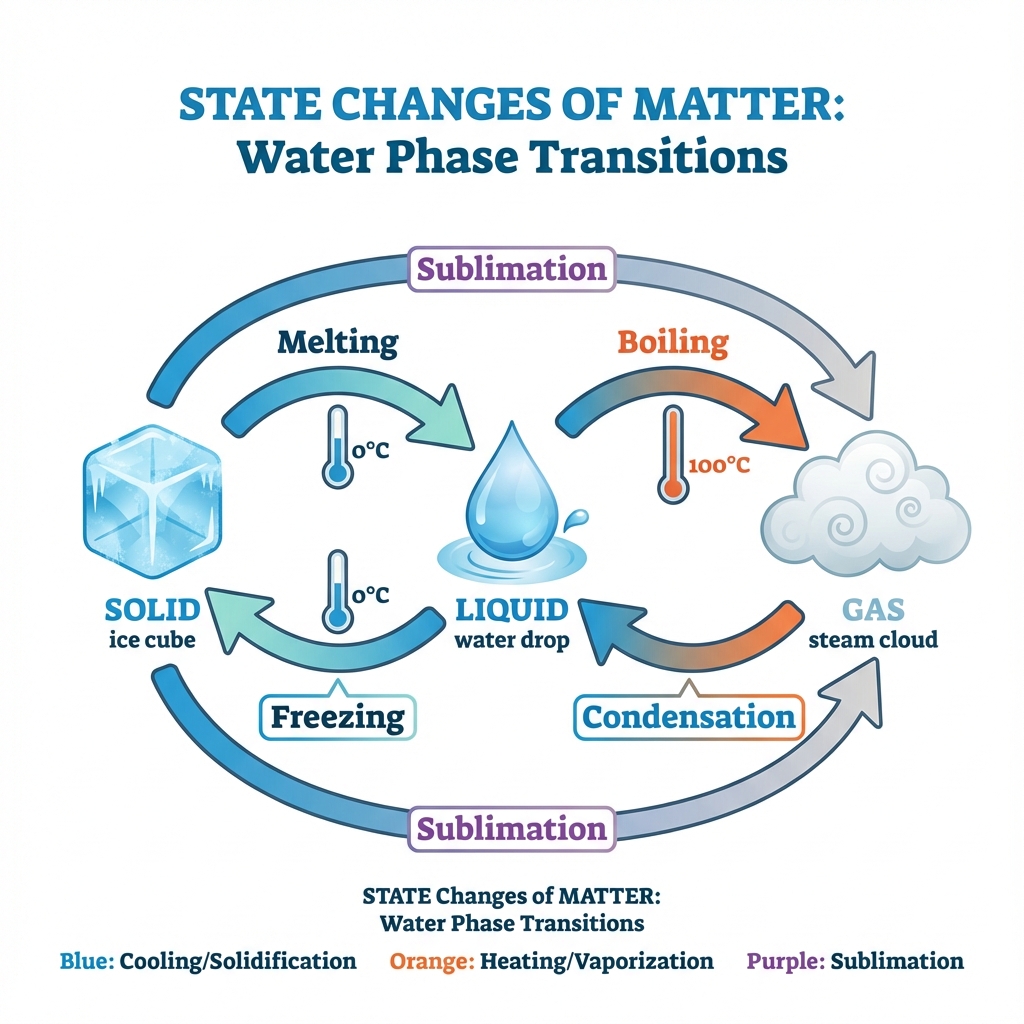
Effect of Temperature 🌡️
🧪 Activity 1.12: Heating Ice
Procedure:
- Take 150g ice in a beaker
- Suspend thermometer with bulb touching ice
- Heat slowly and note temperature
Observations:
| Stage | Temperature | What Happens |
|---|---|---|
| Ice starts melting | 0°C (273 K) | Solid → Liquid |
| All ice melts | 0°C (273 K) | Temperature constant during melting |
| Water starts boiling | 100°C (373 K) | Liquid → Gas |
Key Terms 📚
Melting Point
The minimum temperature at which a solid melts to become liquid at atmospheric pressure.
- Ice melting point: 0°C = 273 K
- The process of melting is also called FUSION
Boiling Point
The temperature at which a liquid starts boiling at atmospheric pressure.
- Water boiling point: 100°C = 373 K
- Boiling is a bulk phenomenon
Latent Heat 🔥
Why does temperature stay constant during state change?
The heat energy is used to:
- Overcome forces of attraction between particles
- NOT to increase kinetic energy
This heat is "hidden" and called Latent Heat.
Latent Heat of Fusion
Heat energy required to change 1 kg solid → liquid at melting point
Latent Heat of Vaporisation
Heat energy required to change 1 kg liquid → gas at boiling point
Important Comparison 💡
| State | At Same Temperature | Energy |
|---|---|---|
| Ice at 0°C | Lower energy | Less |
| Water at 0°C | Higher energy | More (absorbed latent heat) |
| Water at 100°C | Lower energy | Less |
| Steam at 100°C | Higher energy | More (absorbed latent heat) |
This is why steam burns are more severe than boiling water burns! 🔥
Temperature Conversion 🔄
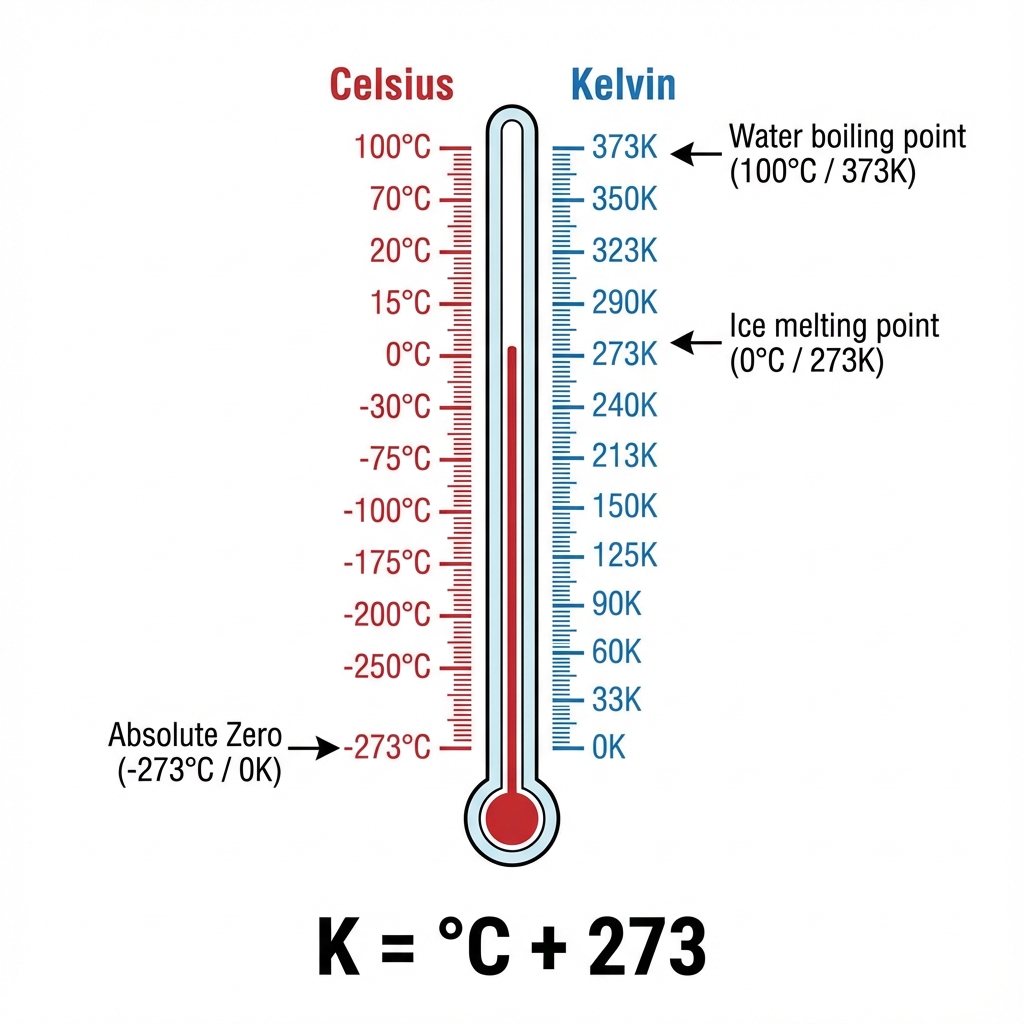
Celsius to Kelvin: Add 273
- 0°C = 273 K
- 100°C = 373 K
Kelvin to Celsius: Subtract 273
- 300 K = 27°C
- 373 K = 100°C